Sponsor Oversight: Vendor Prequalification and Selection
1 – Introduction
Effective vendor oversight is a fundamental element of clinical study governance and a core regulatory expectation across global health authorities. Sponsors are required to demonstrate that all third-party activities are conducted under appropriate supervision, with clear evidence of qualification, continuous monitoring, and quality control. This ensures that study integrity, participant safety, and data reliability are upheld, even when operational tasks are outsourced.
Across all major regulatory authorities, the FDA (United States), EMA (European Union), and MHRA (United Kingdom) Sponsors are legally obligated to maintain full oversight of their clinical studies, including those activities delegated to Contract Research Organisations (CROs) or other service providers.
Despite the delegation of tasks, accountability for study conduct, data integrity, and subject safety cannot be transferred. Sponsors remain ultimately responsible for ensuring that all clinical studies are conducted, monitored, and reported in compliance with regulatory and ethical standards.
Contents
2 – ClinOps Specialists’ Approach
ClinOps Specialists emphasise that maintaining robust, documented oversight of all vendors and service providers is essential not only to meet these regulatory obligations but also to demonstrate control and due diligence during inspections or audits.
A structured and well-documented vendor selection and qualification process forms the foundation of effective oversight. Regulators expect Sponsors to apply a consistent, risk-based approach, supported by objective evidence confirming that each vendor has been assessed for suitability prior to engagement and monitored throughout the relationship.
ClinOps Specialists recommend implementing a transparent and standardised framework covering the entire vendor lifecycle — from initial needs assessment and due diligence through qualification, ongoing performance oversight, and requalification. This framework should document the rationale for vendor selection, the evidence used to confirm capability and compliance, and the methods used to monitor quality, mitigate risks, and ensure accountability throughout the study lifecycle.
The following sections outline the key components of this framework, all of which interlock to produce a robust vendor oversight strategy.
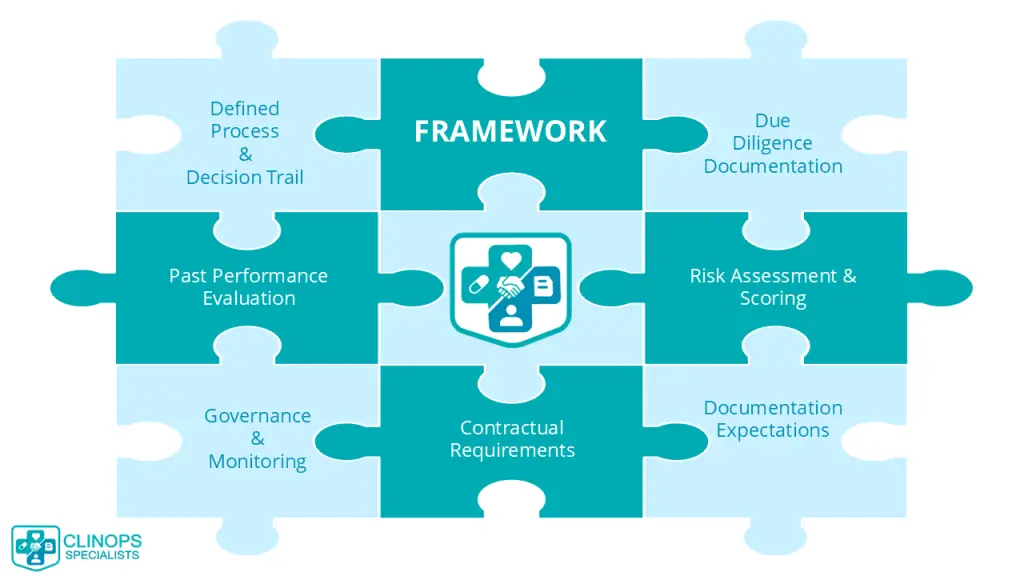
3 – Framework
3.1 – Defined Process and Decision Trail
- a needs assessment to determine which services will be outsourced
- defining selection criteria based on technical expertise
- requirements for quality systems, compliance history, and capacity
3.2 – Due Diligence Evidence
The Sponsor is responsible for performing comprehensive due diligence on each potential vendor. This includes reviewing documentation that supports:
- Vendor capabilities
- company profiles
- service descriptions
- case studies/ evidence of similar projects/complexity
- validated systems; secure data handling)
- Therapeutic experience
- list of prior studies
- clients in similar indications
- Quality Management System (QMS)
- SOP index
- training programs
- governance structure
Evidence also needs to include details for Training & Competency for example; CVs to review, training matrix, role based qualifications, GCP/protocol/system training.
The Sponsor should also examine inspection and audit history (e.g., MHRA/FDA/EMA inspection reports, audit outcomes), Key Performance Indicators (KPIs) (e.g., delivery timelines, data quality metrics), and financial and resource adequacy.
3.2.1 – Financial Assessment
Assessing financial adequacy ensures that a vendor has the stability and resources to meet contractual and operational commitments throughout a clinical study. The Sponsor should review the vendor’s most recent audited financial statements to confirm positive net assets, steady revenue trends, and the absence of “going concern” warnings. Company status and filings should be verified through official registries such as Companies House (UK) or equivalent international databases.
A credit or financial risk report from providers like Dun & Bradstreet or Creditsafe can supplement this review by identifying payment reliability and any adverse financial events. The Sponsor should also confirm resource adequacy, ensuring the vendor has sufficient staffing, infrastructure, and contingency capacity to support the clinical study delivery.
Financial monitoring should continue throughout the contractual engagement, with periodic reassessments or triggers for review if significant organisational or financial changes occur. All findings and conclusions should be documented as part of the vendor qualification to demonstrate due diligence and oversight.
3.2.2 – Conflict of Interest
Conflict Of Interest (COI) checks should be documented through COI declaration forms. A COI Declaration Form should collect details needed to identify and manage any interests that could compromise a vendor’s independence or objectivity. It must include the vendor and individual’s identification details, the clinical study name, and the date and signature.
The form should require disclosure of financial interests (such as investments, payments, or incentives), personal or professional relationships (e.g., with Sponsor staff or investigators), organisational affiliations (such as board memberships or ownerships), and any relationships with subcontractors (subcontractor oversight should be evaluated by requesting the vendor’s subcontractor qualification process or a list of critical third parties they intend to engage).
The vendor/signatory must certify that the information is accurate and agree to disclose new conflicts if they arise. The Sponsor should review and document any identified risks and mitigation measures and file the signed form in the Trial Master File (TMF) as part of oversight evidence.
3.3 – Past Performance Evaluation
It is important for the Sponsor to thoroughly evaluate a vendor’s past performance to ensure reliability, compliance, and capability before engagement. The Sponsor should request and review supporting documentation such as:
- study summaries
- performance metrics dashboards
- inspection or audit outcomes
- client satisfaction feedback
- redacted audit reports
References should be obtained from at least two or three recent Sponsors or CRO partners and verified through structured calls or questionnaires.
The vendor’s regulatory and audit history must be assessed to identify any prior findings and confirm that Corrective and Preventive Actions (CAPAs) have been effectively implemented.
The Sponsor should also review KPIs to gauge operational quality and efficiency, including metrics such as:
- query turnaround times
- site activation timelines
- data entry timeliness
- deviation management
- inspection readiness.
The vendor’s industry reputation should be assessed through clinical trial registries, regulatory authority websites, and professional networks to confirm identity and ensure there are no sanctions or debarments. Finally, it is important for the Sponsor to review their own internal records of prior collaborations to evaluate historical performance, issue logs, and lessons learned, ensuring that previous experiences inform the current qualification decision.
3.4 – Risk Assessment and Scoring
A risk assessment and scoring process helps the Sponsor objectively evaluate and document the level of risk associated with each vendor and determine the level of oversight required before and during engagement. This process should be structured, evidence-based, and applied consistently across all vendors to support transparent qualification decisions.
The Sponsor should begin by defining the evaluation dimensions that will be scored for each vendor. Typical dimensions include:
- Quality and Compliance
- Performance History
- Operational Capacity and Resources
- Financial Stability
- Industry Reputation.
Each dimension should be rated on a consistent scale (for example, 1 to 5, where 1 = poor/high risk and 5 = excellent/low risk). The ratings should be supported by documented evidence gathered during due diligence, such as audit reports, client references, KPIs, and financial statements.
Once each dimension is scored, a weighted scoring system can be applied to reflect the relative importance of each factor. For example, Quality and Compliance might carry 30–40% of the total weight, Performance History 20–25%, Capacity 15%, Financial Stability 15%, and Reputation 10%. These weights can be adjusted depending on the criticality of the service.
The total weighted score will then provide an overall risk rating, which should be categorised as High, Medium, or Low risk based on predefined thresholds (e.g., ≥4.0 = Low risk, 3.0–3.9 = Medium risk, <3.0 = High risk).
3.4.1 – Vendors Audits & Assessments
For high-risk vendors, for example, those providing core GCP services such as monitoring, data management, or pharmacovigilance, ClinOps Specialists recommend a qualification audit before contract award. A qualification audit is conducted to confirm that a vendor’s systems and processes comply with GCP and Sponsor requirements before engagement. It typically reviews the vendor’s QMS, regulatory compliance history, operational procedures, staff qualifications, IT and data integrity controls, and overall facilities or infrastructure.
The audit typically would include:
- document review
- staff interviews
- process walkthroughs to assess quality oversight
- CAPA management
- regulatory readiness
Findings are documented in an audit report with observations categorised by severity, and corrective actions agreed upon. Vendor approval is granted only after all critical and major findings are resolved and CAPAs are verified as effective. All relevant documentation is filed in the TMF.
Medium-risk vendors may be qualified based on a comprehensive questionnaire and document review, with an audit conducted post-award or at study start-up.
Low-risk vendors such as non-critical service providers may only require a basic qualification review or questionnaire.
The Sponsor should also document rationale for each score, including references to supporting evidence and any identified mitigation measures (e.g., closer oversight, additional reporting, CAPA follow-up). All completed risk assessments should be filed in the TMF and reviewed periodically to ensure they remain current, especially if vendor performance, ownership, or service scope changes.
3.5 – Governance and Monitoring
The Sponsor must implement ongoing governance and monitoring to ensure continuous oversight of vendor performance. This can include establishing monthly governance meetings with agreed agendas and actions, maintaining KPI dashboards to track deliverables and quality metrics, and documenting risk and issue logs for timely escalation and resolution.
The Sponsor should also maintain oversight of audit findings and CAPA implementation, review TMF completeness and quality reports, and periodically perform vendor performance reviews summarising compliance and operational trends.
3.6 – Contracts and Statements of Work (SOWs)
The Sponsor must ensure that all vendor contracts and SOWs clearly define the following:
- Project deliverables
- Timelines
- KPIs
- study-specific plans to be created (including ownership and update responsibilities)
- applicable SOPs
- performance expectations
- explicit escalation pathways
- data privacy and security provisions (e.g., GDPR compliance clauses).
If subcontractors are used, the Sponsor must ensure transparency and prior approval are included within the contract. For example, the SOW should specify how often performance reviews will occur, which KPIs will be tracked, and what actions will be taken if performance falls below defined thresholds.
3.7 – Records and Documentation
The Sponsor is responsible for maintaining complete, auditable documentation demonstrating oversight and compliance. This includes:
- signed vendor assessments and qualification reports
- audit reports with CAPA closure evidence
- entries in an Approved Vendor List
- risk assessments
- requalification records.
ClinOps Specialists suggest maintaining all of the above oversight information as a central repository, ‘Vendor Oversight’ within the TMF or QMS to ensure all vendor qualification and oversight activities are well-documented, transparent, and easily retrievable for audit or inspection purposes.
This approach supports regulatory compliance while promoting consistency, accountability, and effective Sponsor oversight and due diligence throughout the vendor lifecycle from identification and qualification to ongoing monitoring and requalification.
