Clinical Study Close-Out
Clinical Study Close-Out activities are essential to wrapping up a study in a compliant and organised manner. This phase ensures that all documentation, data and site obligations are completed, reconciled and archived, and any equipment and investigational products are returned or destroyed, positioning Sponsors for a smooth future submission, audit, or regulatory inspection.
We make your study close-out smooth, compliant, audit- and inspection-ready, so you’re prepared for what comes next.
Close With Confidence. Prepare for Inspection and Dissemination.
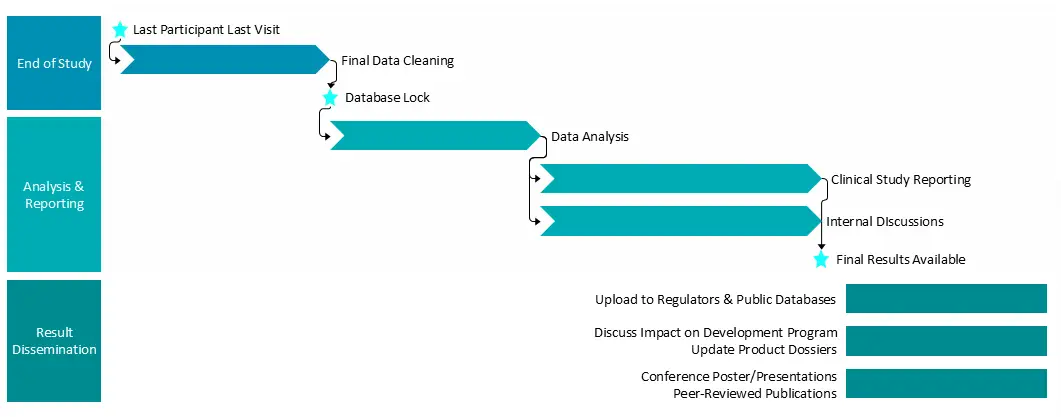
Clinical study close-out typically encompasses the following components:
Close-out Planning & Site Management
- Develop a study close-out plan
- Schedule and conduct site Close-Out Visits
- Perform final reconciliation of source data, Case Report Forms, Investigational Product and regulatory documents
- Deactivation site accounts in Electronic Data Capture, Interactive Web-based Randomisation Systems and other systems
- Document subject status and final disposition
- Notify Ethics Committees and health authorities of study end, as applicable
Audit & Inspection Readiness
- Prepare of audit-ready documentation (Trial Master File, data, correspondence)
- Implement site audit readiness checklists and coaching
- Develop or update audit trail documentation
- Support mock audits or regulatory pre-inspections
- Use Corrective Action Preventive Action (CAPA) tracking for known issues or findings
Electronic Trial Master File (eTMF) Reconciliation
- Conduct completeness check of eTMF content (per Drug Information Association template or Sponsor SOPs)
- Perform eTMF Quality Control for missing, duplicated, or outdated documents
- Agree archiving strategy and system handoff support
Final Reporting & Handover
- Review final monitoring visit reports
- Perform final Ethics Committee and regulatory notifications
- Support Clinical Study Report preparation
- Develop Sponsor handoff and lessons learned documentation
- Support posters and presentations of data at conferences
- Support publication of results in peer reviewed journals
