Study Planning & Start-up
Clinical Study Planning is the foundational phase of the study’s lifecycle.
It includes defining the strategy, operational design, timelines, resources and regulatory roadmap required for successful study execution.
Our extensive experience will ensure that you:
- Lay the foundation for study success through data-driven, risk-aware planning
- Avoid costly delays and surprises with proactive feasibility and recruitment scenario modelling
- Accelerate First Participant In with the right sites, in the right countries, with the right resources
- Proactively engage with all Stakeholders ensuring alignment with all key objectives
From Strategy To Start-up, We Build the Roadmap For Clinical Success.
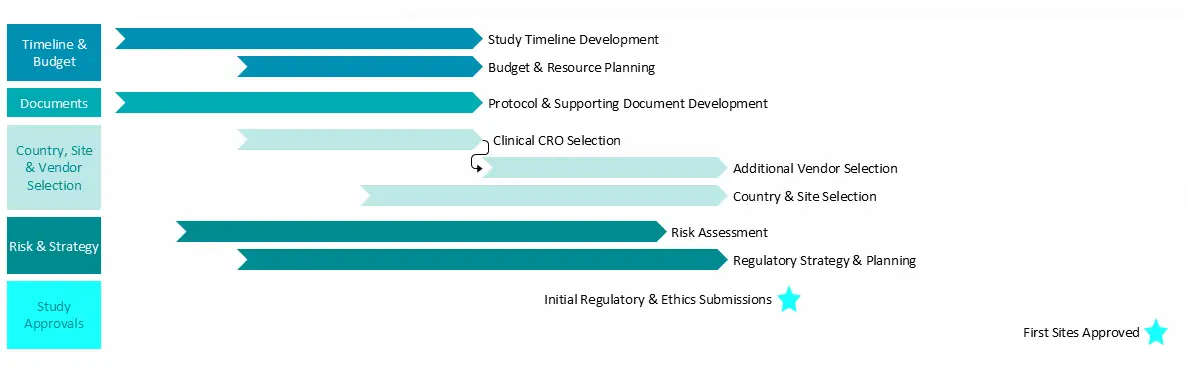
Clinical study planning & start-up typically encompasses the following components:
Protocol Feasibility, Design, and Writing Support
- Advise on operational aspects of protocol design
- Coordinate protocol writing
- Provide input on inclusion/exclusion criteria, endpoints and visit schedules
- Review protocol to ensure operational feasibility
Country & Site Feasibility
- Analyse geographic feasibility based on epidemiology, site experience and regulatory landscape
- Recommend country and site mix
Study Timeline Development
- Define milestones: First Participant In, Last Participant In, database lock, etc.
- Create high-level and detailed project timelines, Project Management Plan and Gantt chart
Budget & Resource Planning
- Forecast study costs: site grants, vendors, internal/external resources
- Develop resource plans for study teams and vendors
Risk Assessment & Mitigation Planning
- Identify risks (e.g., slow enrolment, site readiness) and mitigations
- Develop Risk Management Plan
- Develop risk-based monitoring strategy framework and Co-Monitoring Plan
Vendor Strategy & Sourcing Support
- Identify which services to outsource (e.g., Contract Research Organisation, central labs)
- Draft vendor scope, Request For Proposal and agree selection process
Regulatory Pathway Planning
- Outline submission and approval timelines by country
- Identify early regulatory and ethics considerations
- Agree regulatory submission approach, initial countries and backup countries
Recruitment & Retention Planning
- Build participant journey map (participant pathway) and identify recruitment challenges
- Incorporate recruitment risks into Risk Management Plan
- Draft high-level recruitment strategies and tactics
